Latest research has been published in Nature
Methane Oxidation to Ethanol by a Molecular Junction Photocatalyst

The 18th National Solar Photochemistry and Photocatalysis Conference
Prof. Tang was invited to deliver a Plenary Lecture

2025 Teacher's Day
Students celebrate Teacher's Day with Prof. Tang
Introduction
The Laboratory of photon-phonon co-driven catalysis led by Professor Junwang Tang, has established a new concept of photon-phonon co-driven catalysis, which is employed to achieve efficient conversion of small molecules. In parallel, the group also focuses on plastic waste recycling by microwave catalysis and biomass transformation through multi-energy-coupled catalytic technologies.
The group's main research projects include: ammonia synthesis, hydrogen production, biomass conversion, plastic depolymerization, natural gas conversion and multi-scale ultrafast spectroscopy.
Research
Ammonia synthesis
1. To develop advanced thermal catalysts for efficient ammonia synthesis under mild conditions, aiming to overcome the energy-intensive limitations of the Haber-Bosch process through innovative catalyst design.
2. To pioneer photon-phonon co-driven catalytic systems that synergistically combine light and thermal energy for efficient nitrogen fixation. This enables ammonia synthesis from nitrogen and water (or hydrogen) at significantly lower temperatures and pressures than conventional methods, offering a sustainable pathway toward decentralized green ammonia production.

Solar-driven hydrogen production
To design highly efficient and stable photon-phonon co-driven catalytic systems to achieve solar-driven water splitting or methanol reforming for green hydrogen production, thereby contributing to the development of the hydrogen energy industry and the realization of carbon neutrality goals.

Photon-phonon co-driven catalysis for biomass conversion
To develop an efficient photon–phonon co-driven catalysis strategy for the selective and efficient conversion of biomass, including the conversion of glycerol into glyceraldehyde, glycolaldehyde, or lactic acid and methanol into value-added chemicals under mild conditions.
Microwave-assisted catalysis for waste recycling
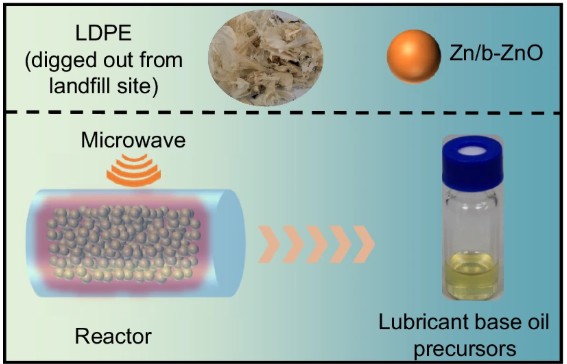
To employ microwave-catalysis technology for the efficient degradation and conversion of waste plastics (e.g., PE, PP, PET), enabling the targeted production of high-value chemicals or fuels to promote white pollution control and carbon resource recycling.
Photon-phonon co-driven catalysis for natural gas conversion
To develop novel catalytic materials and reaction systems for efficient conversion of natural gas into high-value chemicals or clean fuels at low temperatures, offering a low carbon route for natural gas resource utilization.

Multi-scale in-situ spectroscopy
To develop the in-situ/ operando multifunctional time-resolved (fs-ms) spectroscopies, aiming to unveil the reaction pathways in thermal catalysis, electrocatalysis and photon-phonon co-driven catalysis.

Members
Professor

Professor Tang is a pioneer in employing multiple energy sources to activate small molecules (e.g., H2O, N2, CH4, CO2). He develops low carbon catalytic systems for the conversion of these molecules to high-value chemicals and employs microwave catalysis to depolymerize solid waste, enabling resource recycling. He is also dedicated to investigating the reaction mechanisms of photo/thermal/electro-catalysis using multi-modal time-resolved spectroscopies.
Professor Tang has authored more than 250 papers in prestigious international journals such as Nature, Nature Catalysis, Nature Energy, Nature Materials, and Nature Sustainability. His work has been cited over 36,000 times, earning him an h-index of 94 (Google Scholar). He has been granted 25 patents and has been recognized as a Clarivate Analytics Highly Cited Researcher on multiple occasions. He also serves as an Editor or Associate Editor for 6 international journals, including Applied Catalysis B: Environment and Energy, Chinese Journal of Catalysis, and Energy & Environmental Science: Solar.
Google Scholar link: https://scholar.google.com/citations?user=S0jdSPEAAAAJ&hl=zh-CN&oi=sra
Postdoctoral Researchers

Photon-phonon co-driven catalysis for ammonia synthesis

Photon-phonon co-driven methane conversion

Photon-phonon co-driven catalysis for methanol conversion

Thermal catalysis for ammonia decomposition

Mechanistic investigation of photothermal catalytic systems using multi-scale time-resolved spectroscopy

Methane conversion, ammonia synthesis, and plastic conversion

Photon-phonon co-driven oxidation of nitrogen to nitrite

Microwave catalysis for convertion of waste plastics into high-value products

H2O2 synthesis coupled with biomass conversion

Photocatalytic ammonia synthesis

Thermal catalytic green ammonia synthesis

Organic small molecule amidation

Moiré superlattice materials, nitrogen fixation and scattering techniques (XPDF, XAS, SXRD, and NPD) for investigating catalysts
Doctoral candidates
Photon-phonon co-driven CO2 conversion

Photon-phonon co-driven catalytic NH3 synthesis

Photon-phonon co-driven methane conversion

Photon-phonon co-driven methanol conversion

Photon-phonon co-driven ammonia synthesis

Photon-phonon co-driven C-H activation

Ultrafast spectroscopic analysis of carrier dynamics in photon-phonon co-driven catalysis

Time-resolved molecular vibration spectroscopy

Photon-phonon co-driven catalytic C-F bond activation
Undergraduates

Photon-phonon co-driven ammonia synthesis from N2 and H2O
Alumni

Energy and environmental photocatalysis with particular emphasis on the photocatalytic conversion of methane

Light-/Thermal-driven small molecule conversion
Publications in the Past 10 Years
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
2015
2014
All Publications
News
Our group published a research paper in Advanced Materials: Infrared and Thermo Co-Driven Catalysis for CO2 Conversion to Valuable Chemicals
Our group has achieved a breakthrough in converting CO2 into dimethyl carbonate (DMC). By utilizing a novel photon-thermal synergy process with a defect-engineered, noble-metal-free cerium oxide catalyst, we have attained a remarkable DMC production rate of 30 mmol/g/h with 100% selectivity. This work overcomes the performance bottlenecks of conventional thermal catalysis and introduces a new strategy of IR-driven catalysis for CO2 utilization.
Link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202512626



The research group achieves fruitful results at the 18th National Solar Photochemistry and Photocatalysis Conference
Our group achieved fruitful results at the 18th National Conference on Solar Photochemistry and Photocatalysis held in Tianjin. Professor Tang, Junwang present a Plenary Lecture titled "From Photocatalysis to Photon-Phonon Co-Driven Catalysis," which garnered significant attention from attending experts. Dr. Xiong, Lunqiao presented an oral presentation in a parallel session entitled "Application of Single-Atom/Atom Cluster Photocatalysts in Glycerol Oxidation." Furthermore, the academic poster presented by Zheng, Yaxuan, received the Best Poster Award.
Link: https://news.tju.edu.cn/info/1012/566149.htm



Professor Junwang Tang was awarded the International Science Award for Crossing Boundaries by Falling Walls Foundation
Professor Junwang Tang has been named a 2025 Falling Walls Breakthrough Winner in Engineering and Technology. This prestigious award, presented by Germany's Falling Walls Foundation, honors top scientific breakthroughs selected from more than 1,500 international nominations.
Link: https://falling-walls.com/engineering-technology
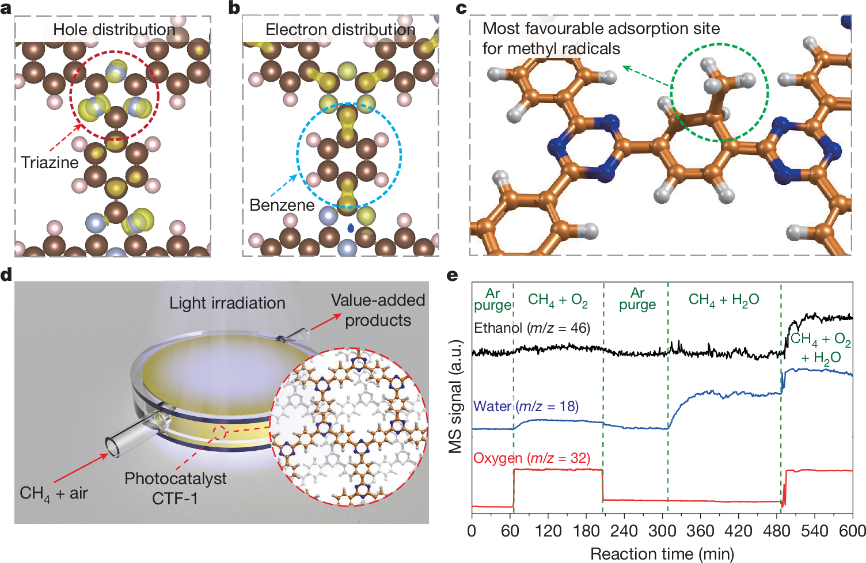
Our group published a research paper in Nature: Methane Oxidation to Ethanol by a Molecular Junction Photocatalyst
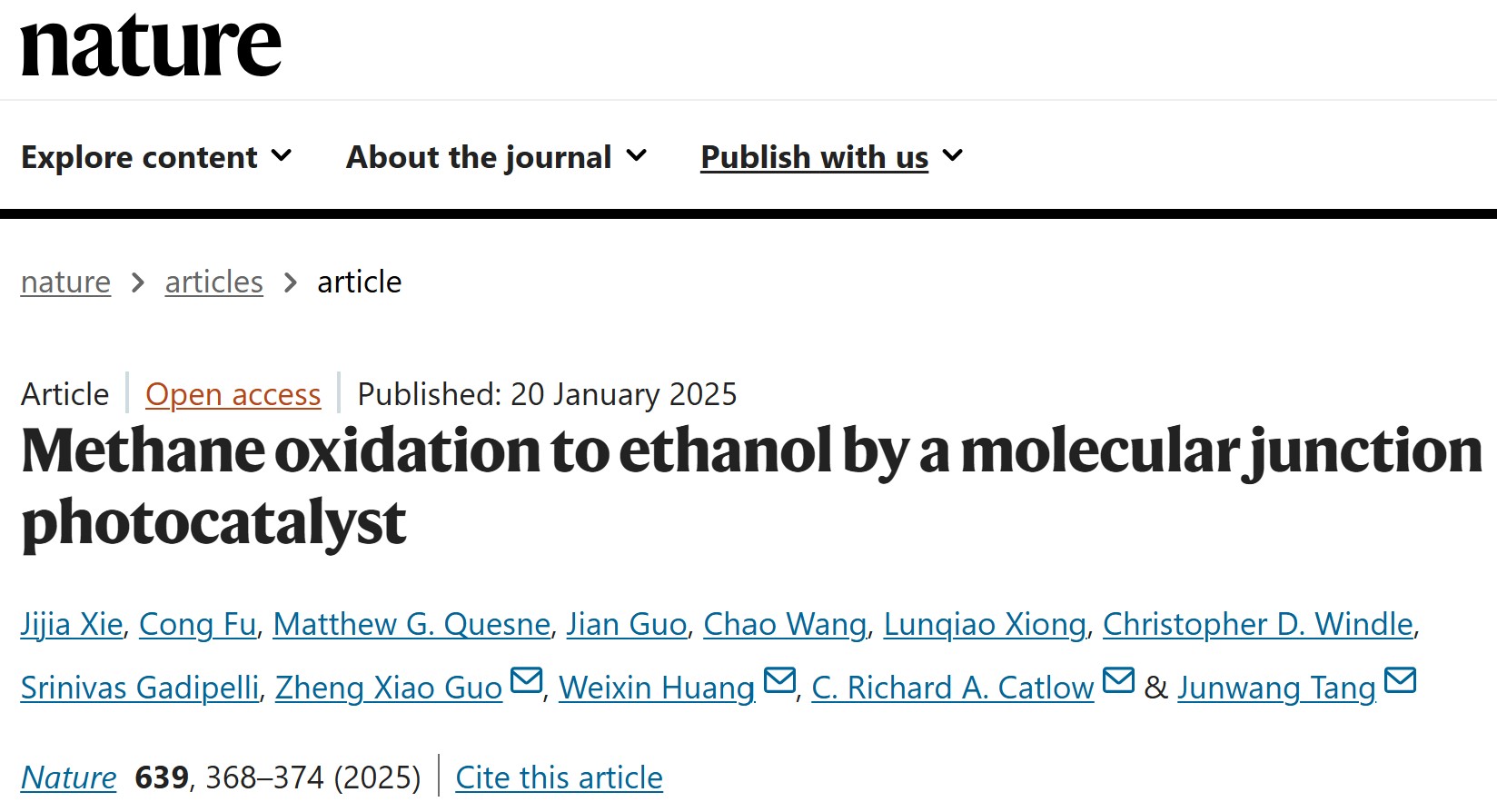
Professor Tang Junwang's team proposed a novel concept of molecular junction and developed the CTF-1 catalyst. Leveraging the synergy between triazine and benzene ring units, this catalyst enables the highly efficient, one-step conversion of methane to ethanol at room temperature and pressure under light irradiation. This technology provides a groundbreaking pathway for the high-value utilization of natural gas resources, which not only helps reduce the chemical industry's heavy reliance on petroleum but also contributes to achieving carbon neutrality goals.
This achievement is the result of nearly a decade of highly efficient collaboration among a multinational team comprising five universities from China and the UK, highlighting the essential role of global scientific cooperation in addressing critical energy challenges. The related research, entitled "Methane oxidation to ethanol by a molecular junction photocatalyst," was published online in Nature on January 20 (Nature 2025, 639, 368–374).
Link: https://www.nature.com/articles/s41586-025-08630-x
Equipment
Our laboratory is equipped with state-of-the-art facilities for thermal, photon-phonon co-driven and microwave-assisted catalysis research. Some are listed below.
Ammonia Synthesis/Decomposition

Ammonia synthesis reaction evaluation devices

Gas compression device

Photon-phonon co-driven ammonia synthesis reaction system

Thermal catalytic ammonia decomposition reaction system

Photon-phonon co-driven ammonia synthesis reaction system

Photon-phonon co-driven nitrogen oxidation reaction system

Photon-phonon co-driven ammonia synthesis reaction system

Photon-phonon co-driven ammonia synthesis reaction system

Photon-phonon co-driven ammonia synthesis reaction system with 50×sun light intensity Xe light source

Ultrasonic-photon coupled catalytic ammonia synthesis reaction system

Ion chromatography
Solar-driven Hydrogen Production

Photon-phonon co-driven methanol reforming reaction system

Photon-phonon co-driven methanol reforming reaction system
Gas chromatography
Photon-phonon Co-driven Biomass Conversion

Photon-phonon co-driven methanol conversion reaction system

Photon-phonon co-driven glycerol conversion device

Gas chromatography-mass spectrometry
Microwave-assisted Catalysis of Plastic Depolymerisation

Microwave assisted catalysis of plastic degradation reaction system
Photon-phonon Co-driven Natural Gas Conversion



3 setups for photo-phonon co-driven natural gas conversion

Phton-phonon co-driven methane combustion evaluation device

Gas chromatography

Gas chromatography
Multi-scale Time Resolved Spectroscopy


Time-resolved ultrafast spectroscopy platform for photophysics and reaction kinetics analysis [transient absorption spectrometer (TAS); time-resolved infrared spectrometer (TRIR); femtosecond stimulated Raman spectrometer (FSRS)]
Materials Synthesis and Characterisation

Uv-vis-nir absorption spectrometer

Photoluminescence spectrometer

Tube furnace

Air drying oven

Air drying oven

Vacuum oven

Vacuum oven

Muffle furnace

Centrifuge
Galleries











